In May 2009, the United States Food and Drug Administration issued a Warning Letter to Johnson & Johnson, alleging that a promotional website commissioned by the manufacturer had "overstated the efficacy" of the drug, and "minimized the serious risks". The company which produced it, the German pharmaceutical company Grünenthal GmbH, were alleged to be guilty of "minimizing" the habituating nature of the drug, although it showed little abuse liability in preliminary tests.[citation needed] The 2010 Physicians Desk Reference contains several warnings from the manufacturer, which were not present in prior years. The warnings include stronger language regarding the habituating potential of tramadol, the possibility of difficulty breathing while on the medication, a new list of more serious side effects, and a notice that tramadol is not to be used in place of opiate medications for addicts. Tramadol is also not to be used in efforts to wean addict patients from opiate drugs, nor to be used to manage long-term opiate addiction.
Availability and usage

100mg tramadol injection, marketed by the original 'Contramal' trade-mark owner, Grünenthal GmbH.(Hungarian release)
50 mg Tramadol HCl tablets (generic Ultram) marketed by Akyma Pharmaceuticals. Immediate release tramadol HCl is available in many generic preparations.
Bottle of 30 tablets of 200 mg extended-release tramadol HCl (generic Ultram ER) marketed by Patriot. Extended-release tramadol is commonly available in 100, 200, and 300 mg strengths to be taken once daily. It is often prescribed with tramadol-APAP (Ultracet) or regular immediate-release tramadol HCl (Ultram/Tramal/Rybix) for breakthrough pain

Tramadol HCl for injection
Tramadol comes in many forms, including:
- capsules (regular and extended release)
- tablets (regular, extended release, chewable, low-residue and/or uncoated tablets that can be taken by the sublingual and buccal routes)
- suppositories
- effervescent tablets and powders
- ampules of sterile solution for SC, IM, and IV injection
- preservative-free solutions for injection by the various spinal routes (epidural, intrathecal, caudal, and others)
- powders for compounding
- liquids both with and without alcohol for oral and sub-lingual administration, available in regular phials and bottles, dropper bottles, bottles with a pump similar to those used with liquid soap and phials with droppers built into the cap
- tablets and capsules containing (acetaminophen/APAP), aspirin and other agents.
Tramadol has a characteristic and unpleasant taste which is mildly bitter but much less so than morphine and codeine. Oral and sublingual drops and liquid preparations come with and without added flavoring. Also, 50 mg water-soluble tramadol tablets has strawberry-flavouring, no matter which company manufactured it, to distinguish every, same-looking and same sized Mirtazapine sublingual tablets, which has orange flavouring irrespective of the manufacturer. This different flavouring is considered to be a standard. Its relative effectiveness via transmucosal routes (i.e. sublingual, buccal, rectal) is similar to that of codeine, and, like codeine, it is also metabolized in the liver to stronger metabolites (see below).
The maximum dosage per day is 400 mg for oral use and 600 mg for parenteral use. Certain manufacturers or formulations have lower maximum doses. For example, Ultracet (37.5 mg/325 mg tramadol/APAP tablets) is capped at 8 tablets per day (300 mg/day) due to its acetaminophen content. Ultram ER is available in 100, 200, and 300 mg/day doses and is explicitly capped at 300 mg/day as well.
Patients taking SSRIs (Prozac, Zoloft, etc.), SNRIs (Effexor, etc.), TCAs, MAOIs, or other strong opioids (oxycodone, methadone, fentanyl, morphine), as well as the elderly (> 75 years old), pediatric (< 18 years old), and those with severely reduced renal (kidney) or hepatic (liver) function should consult their doctor regarding adjusted dosing or whether to use tramadol at all.
Investigational uses
- diabetic neuropathy
- antidepressant
- postherpetic neuralgia
- acute opioid withdrawal management
- antidepressant withdrawal aid (proven to be effective, especially with withdrawal from its distant relative venlafaxine (Effexor)).[citation needed]
- obsessive-compulsive disorder
- premature ejaculation
Adverse effects
| Effect | Probability (%) |
|---|---|
| Any adverse effect | 71 |
| Drowsiness | 17 |
| Nausea | 17 |
| Dizziness | 15 |
| Constipation | 11 |
| Headache | 11 |
| Vomiting | 7 |
| Diarrhea | 6 |
| Dry Mouth | 5 |
| Fatigue | 5 |
| Indigestion | 5 |
| Seizure | <1 |
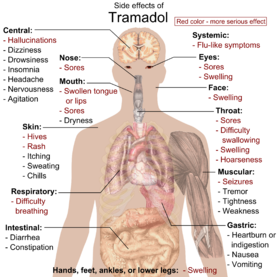
Main side effects of tramadol. Red color denotes more serious effects, requiring immediate contact with health provider.
Fewer than 1% of users have a presumed incident seizure claim after their first tramadol prescription. Risk of seizure claim increases two- to six-fold among users adjusted for selected comorbidities and concomitant drugs. Risk of seizure is highest among those aged 25–54 years, those with more than four tramadol prescriptions, and those with a history of alcohol abuse, stroke, or head injury. Dosages of warfarin may need to be reduced for anticoagulated patients to avoid bleeding complications. Constipation can be severe especially in the elderly requiring manual evacuation of the bowel.[citation needed] Furthermore, there are suggestions that chronic opioid administration may induce a state of immune tolerance, although tramadol, in contrast to typical opioids may enhance immune function. Some have also stressed the negative effects of opioids on cognitive functioning and personality.
Physical dependence and withdrawal
Long-term use of high doses of Tramadol may be associated with physical dependence and a withdrawal syndrome. Tramadol causes typical opiate-like withdrawal symptoms as well as atypical withdrawal symptoms including seizures. The atypical withdrawal symptoms are probably related to tramadol's effect on serotonin and norepinephrine re-uptake. Symptoms may include those of SSRI discontinuation syndrome, such as anxiety, depression, anguish, severe mood swings, aggressiveness, brain "zaps", electric-shock-like sensations throughout the body, paresthesias, sweating, palpitations, restless legs syndrome, sneezing, insomnia, vivid dreams or nightmares, micropsia and/or macropsia, tremors, and headache among others. In most cases, tramadol withdrawal will set in 12–20 hours after the last dose, but this can vary. Tramadol withdrawal lasts longer than that of other opioids; seven days or more of acute withdrawal symptoms can occur as opposed to typically three or four days for other codeine analogues. It is recommended that patients physically dependent on pain killers take their medication regularly to prevent onset of withdrawal symptoms and this is particularly relevant to tramadol because of its SSRI and SNRI properties, and, when the time comes to discontinue their tramadol, to do so gradually over a period of time that will vary according to the individual patient and dose and length of time on the drug.Psychological dependence and recreational use
Some controversy regarding the abuse potential of tramadol exists. Grünenthal has promoted it as having a lower risk of opioid dependence than traditional opioids, claiming little evidence of such dependence in clinical trials (which is true; Grünenthal never claimed it to be non-addictive). They offer the theory that, since the M1 metabolite is the principal agonist at μ-opioid receptors, the delayed agonist activity reduces abuse liability. The norepinephrine reuptake inhibitor effects may also play a role in reducing dependence.Rarely, dependence may occur after as little as three months of use at the maximum dose—generally depicted at 400 mg per day. However, both physicians and health authorities generally consider dependence liability relatively low. Thus, tramadol is classified as a Schedule 4 Prescription Only Medicine in Australia, and been rescheduled in Sweden rather than as a Schedule 8 Controlled Drug like opioids. Similarly, unlike opioid analgesics, tramadol is not currently scheduled as a controlled substance by the U.S. DEA. However, it is scheduled in certain states. Nevertheless, the prescribing information for Ultram warns that tramadol "may induce psychological and physical dependence of the morphine-type". Using tramadol as recreational drug may also be preferred because it is one of the only opioids that is often not screened for by standard urine drug-tests.
Dependence on tramadol has been reported to be a major social problem in the Gaza Strip. The Hamas government has attempted to cut off supplies of the drug, and in April 2010 burnt 2 million tablets which had been intercepted while being smuggled into the territory.
Because of the possibility of convulsions at high doses for some users, recreational use can be very dangerous. However, via agonism of μ opioid receptors, Tramadol can produce effects similar to those of other opioids (codeine and other weak opioids). Due to tramadol's much lower affinity for this receptor, these are not nearly as intense as with the opiates per se. Tramadol can cause a higher incidence of nausea, dizziness, loss of appetite compared with opiates, which could deter abuse. Tramadol can alleviate withdrawal symptoms from opiates, and it is much easier to control its usage than for street drugs. It may also have large effect on sleeping patterns and high doses may cause insomnia. (Especially for those on methadone, both for maintenance and recreation. Though there is no scientific proof tramadol lessens effects of opiates or is a mixed agonist-antagonist, some people get the impression it is, while someone else might benefit being prescribed both for pain and breakthrough pain.









0 comments:
Post a Comment